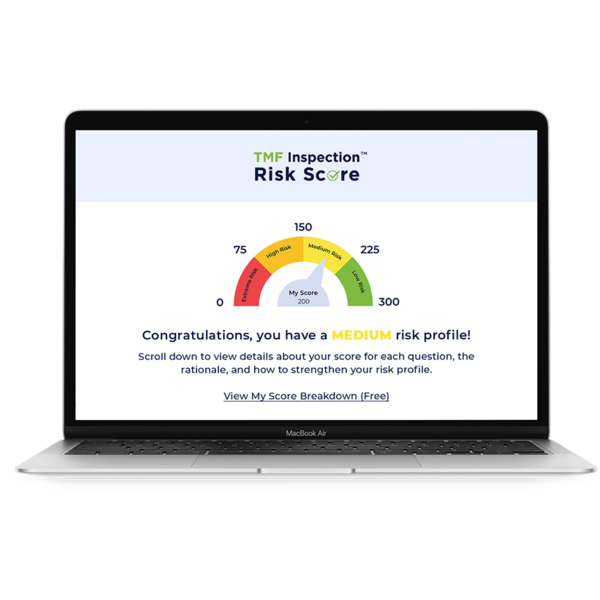
WATCH THE FREE WEBINAR: Risk-Based CAPA Under ICH E6(R3)
Fast-Track Clinical Trial Data Initiation and Maintain Control
Choose from specialized solutions that help you increase efficiency and ensure data integrity in your eClinical platform.

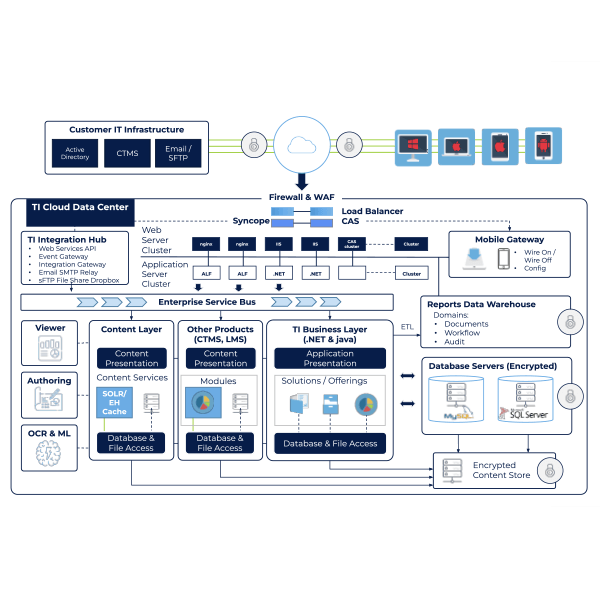
An Efficient Way to Launch and Manage Your Global IT Operations
A suite of solutions that is designed to create robust data collection, adherence to regulations, and timely approvals. With Trial Interactive, you can focus on industry trends and ensure data security throughout the clinical process.