AI in Clinical Trials: From Webinar Insights to Audit-Ready Implementation
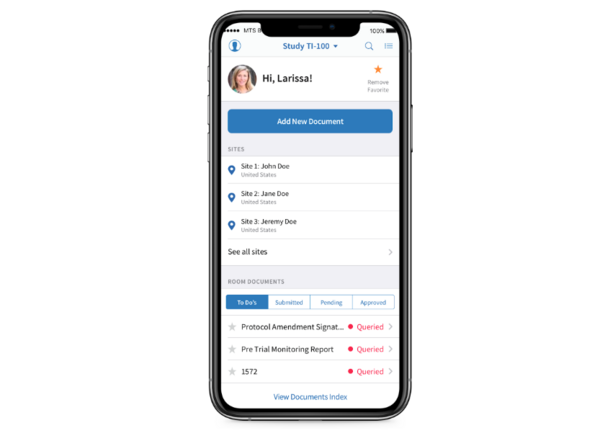
Over three weeks, my colleagues and I hosted a webinar series on AI in clinical trials. The engagement shocked us: when we polled attendees in our final session, 50% reported already using AI in daily operations. This technology has moved from theory to operational reality faster than anyone predicted.