WATCH THE FREE WEBINAR: Risk-Based CAPA Under ICH E6(R3)
Make Faster Risk-Based Decisions and Reduce Cycle Times with Trial Interactive CTMS

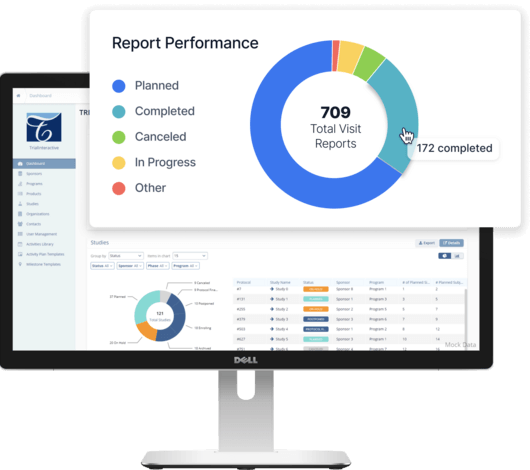
Fast Implementation. Easy to Navigate. Unmatched Process Visibility.
Our next-generation clinical trial management system (CTMS) empowers every stakeholder—from CRAs and study managers, to IT teams and business leadership—to be more efficient. By simplifying study planning and oversight, TI CTMS software removes the friction from day-to-day clinical operations. Learn how you can unlock real-time insights, mitigate global regulatory compliance risks, and accelerate therapeutic breakthroughs with a modern CTMS.
Discover 360° Clinical Trial Management
Manage your CTMS anytime, anywhere.
Designed to be mobile-first, TI CTMS software allows CRAs to perform critical tasks and oversight on the go.
Go live faster with quick setup and implementation.
Our CTMS system is a highly configurable system that can adapt to your business processes and comes with 24/7 global support.
Achieve real-time visibility.
Our CTMS software enables study teams to avoid delays in decision making that lengthen timelines.
Avoid audit nightmares.
Mitigate compliance risks with system-level controls and a real-time audit trail that captures task, data, and correspondence.
Improve operational excellence and efficiencies.
Get a single-source of truth for planning, tracking, and reporting on all your study data and documents.
Achieve a seamless eClinical experience.
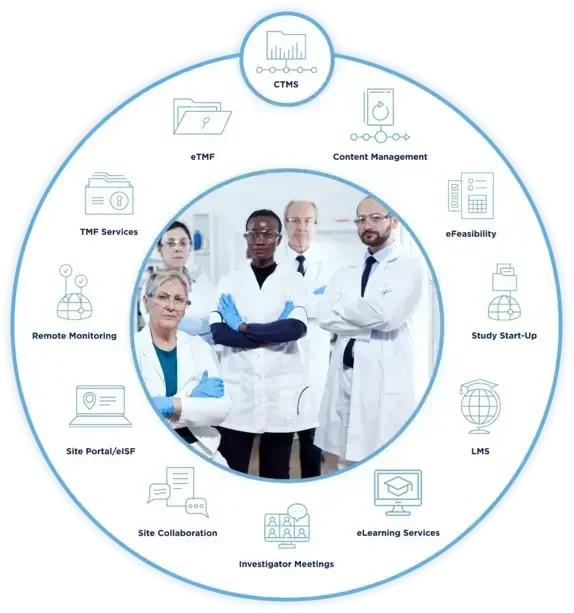
Our CTMS software enables remote oversight and powerful interoperability with site feasibility, study start-up, eTMF, LMS, content management, and collaboration.
Essential Features for a Modern Mobile-Friendly CTMS System
Planning & Management
- Activity Plans with Milestones
- Visual Reporting through Dashboards
- Clinical Portfolio, Program, and Product Management
- Country and Region Management
- Subject and Subject Visit Management
- Flexible Support for Many Trial Designs
- Site Activation and Study Start-Up
Insight & Audit
- Author-to-Archive Site Portal to eTMF
- Letters and Report Creation
- Clinical Trial Performance and Reporting
- Sponsor, Site, Vendor, and Lab Management
- Configurable Reports with Custom Fields
Monitoring & Compliance
- 21 CFR Part 11 and EU Annex 11 Compliant
- Mobile App for Site Management and Monitoring Activities
- Better Data Quality through Standard Business Practices
- Protocol Deviation and Issue Tracking
- Built-in, Best-in-Class Content and eTMF
- Document Workflows with eSignature Approvals
CTMS Seamlessly Connects to our 21 CFR Part 11 Compliant eClinical Platform
Study Management
Dashboards with oversight and visualizations on enrollment, milestones, activities, documents, and plans. Request a Demo >
Study View provides a clear layout of countries, sites, organizations, contacts, activities, milestones, plans, documents, and much more. Request a Demo >
Milestones for a simple view of the overall study, showing both planned and actual milestones by date, priority, and risk. Request a Demo >
Manage the study team within the TI CTMS, simplifying access rights. A comprehensive set of metadata may be defined in the study for a single source of truth. Request a Demo >
Publish and apply activity plan templates across multiple countries, sites, studies, and subjects. Request a Demo >
Site Management
Dashboards with visual insights on subjects, enrollment, milestones, activities, documents, and plans. The study manager can assess deviation or safety trends, visit report cycle times, and other key performance indicators (KPIs). Request a Demo >
Get a clear layout of regions, site users, organizations, contacts, activities, milestones, plans, documents, communications, tasks, deviations, issues, and much more with the Site View. Request a Demo >
Manage activities and activity plans or centrally publish to the site from a standard set of templates. Request a Demo >
View progress with visit schedules, planned and completed activities, protocol information, ICF tracking, deviations, and safety reports. Trend screening failures, protocol visit windows, and required activities. Request a Demo >
Track site status, including oversight of the site selection process and study start-up status. Request a Demo >
Manage site contact records for communication logs, site roles, IP and supply shipment addresses, and CRA assignments. Request a Demo >
Track site documents, upload and reference critical documents, and seamlessly publish to the TI eTMF. Request a Demo >



