WATCH THE FREE WEBINAR: Risk-Based CAPA Under ICH E6(R3)
TI 10.5 Highlights
-
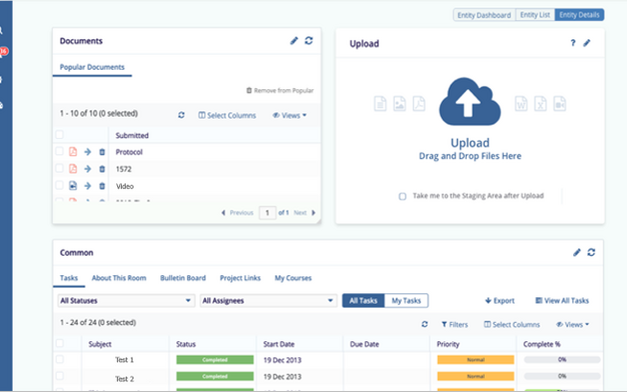
Decrease Study Start-Up and cycle times with a seamless Study Start-Up module
-
Improve Clinical Trial Health with milestone and event triggers that automatically deploy Expected Document Lists to the eTMF
-
Set up rules for creating document relationships that simplify TMF completeness and link critical information
-
Many new options for placeholder assignment: on upload, during health checks prompted within completeness and event views
-
Simplify access with more Part 11 compliant and flexible options for single sign-on, multi-factor authentication, and digital eSignatures
-
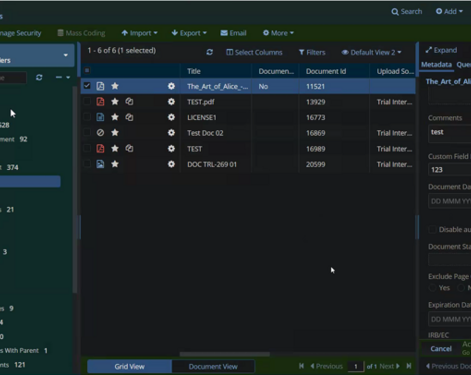
With TI AutoMate, set confidence thresholds for finalizing ML indexed documents automatically