How to Prepare for ICH E6(R3) Guideline for Good Clinical Practice
JOIN US IN LONDON FOR TRIAL INTERACTIVE’S CUSTOMER SUMMIT— OPTIMIZE 2025!
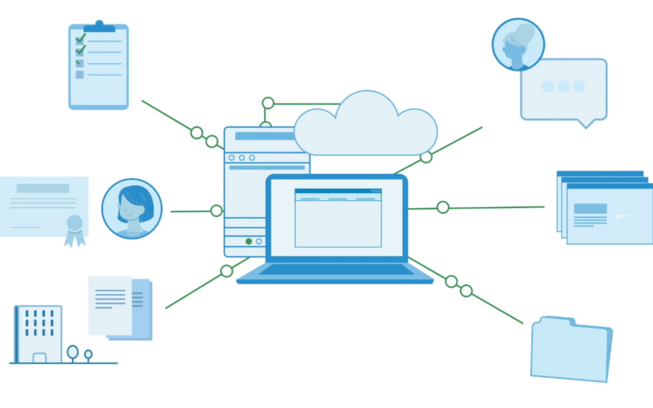
The Enterprise Path to Inspection Readiness
Create, review, and archive clinical content. Launch compliance focused training. Index all required records in the eTMF
-
All within one system
-
Make it easy to show the story of your study to regulatory agencies.